Background: Aplastic anemia (AA) is mediated by T cell destruction of hematopoietic stem and progenitor cells causing pancytopenia and bone marrow hypoplasia. The current standard of care for severe aplastic anemia (SAA) is hematopoietic stem cell transplantation (HSCT) or immunosuppressive therapy (IST) with antithymocyte globulin (ATG) plus cyclosporine (CsA) with or without eltrombopag (Epag), a thrombopoietin receptor agonist. HSCT is preferred for SAA in young adults as a curative option, but only a small proportion of patients have histocompatible siblings and mortality increases with age. The IST plus CsA combination with or without Epag has led to survival rates comparable with those observed with HSCT recipients. However, complete hematologic remission is rare with IST and there is a considerable risk of clonal complications, including PNH evolution and progression to MDS. The aim of this study was to evaluate outcomes of ATG plus CsA therapy in a large cohort of SAA patients.
Methods: We retrospectively analyzed medical records of 156 patients with SAA, aged ≥2 years treated with ATG plus CsA with or without Epag at our institution between July 2000 and January 2023. Hematologic responses were evaluated at 3-, 6- and 12-months post therapy initiation. Super response (SR) was defined as hemoglobin ≥12 g/dL, platelet count ≥150x10 9/L and absolute neutrophil count (ANC) ≥1.0x10 9/L; complete response (CR) was defined as hemoglobin ≥10 g/dL, platelet count ≥100x10 9/L and ANC ≥1.0x10 9/L. Partial response (PR) was defined as not meeting the criteria for CR and SAA. Overall response encompassed SR, CR, and PR. Statistical analyses were performed with the use of Chi-squared test and Kaplan Meier method.
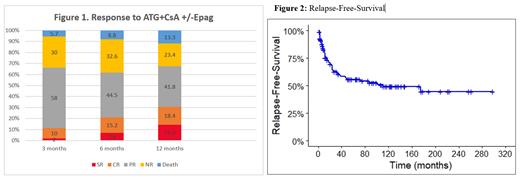
Results: The median age at diagnosis was 52 (IQR 30-66) years, 47% were male, and 86% were Caucasian. Twenty three percent of the cohort had coexisting paroxysmal nocturnal hemoglobinuria. Most patients were treated with ATG plus CsA (92%), the remaining 8% additionally received Epag. Overall response rates were 70%, 67%, and 77% at 3-, 6- and 12-months post-treatment initiation, respectively. CR was achieved in 10%, 15%, and 18% at 3-, 6- and 12-months, respectively. SR was achieved in 16.3% of patients at 12 months, as shown in Figure 1. Patients who achieved SR were young with median age at diagnosis of 28 years, while those who achieved PR or no response had a median age of 54 and 56 years, respectively. Patients who responded to the ATG + CsA therapy had significantly higher baseline ANC (p=0.04), absolute reticulocyte count (ARC) (p<0.001) and absolute lymphocyte count (ALC) (p<0.001). Pre-treatment median values of ANC, ARC and ALC were higher for patients who achieved SR compared to the rest of responders. With a median follow up time of 4.5 years, the estimated median relapse free survival was 9.2 years (95% CI, 3.2 - not reached [NR]) (Figure 2). Of the entire cohort, 21 (13%) patients progressed to acute myeloid leukemia or myelodysplastic syndrome. At data cut off, a total of 66 patients had died; the estimated median OS was 11.6 years (95% CI, 8.4 - NR). In particular, 30 patients died within the first 12-months, 13 within 3-months of ATG initiation from treatment-related complications, 6 from pneumonia and the rest from unknown causes.
Conclusion: The findings of our study demonstrate that ATG plus CsA is an effective and safe treatment option for patients with SAA, especially in the older patient population where HSCT-related toxicities can lead to high mortality. Those with higher baseline ALC, ANC and ARC were more likely to respond to therapy compared to those with lower counts. A small percentage of patients progressed to myeloid neoplasia. Mortality within the first 12-months was high, indicating the need for close monitoring to avoid treatment-related mortality.
Disclosures
Maciejewski:Alexion: Membership on an entity's Board of Directors or advisory committees; Regeneron: Consultancy, Honoraria; Novartis: Honoraria, Speakers Bureau; Omeros: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal